facilities and skills
X-ray diffractometry

The discovery of X-rays in 1895 enabled scientists to probe crystalline structure at the atomic level. X-ray diffraction has been in use in two main areas, for the fingerprint characterization of crystalline materials and the determination of their structure. Each crystalline solid has its unique characteristic X-ray powder pattern which may be used as a "fingerprint" for its identification. Once the material has been identified, X-ray crystallography may be used to determine its structure, i.e. how the atoms pack together in the crystalline state and what the interatomic distance and angle are etc. XRD analysis provides qualitative and quantitative information about the crystallographic order in relation to the composition of the sample, the cell parameters and the average size of the crystallites. X-ray diffraction is one of the most important characterization tools used in solid state chemistry and materials science. We can determine the size and the shape of the unit cell for any compound most easily using X-ray diffraction.
X-ray diffraction is based on constructive interference of monochromatic X-rays and a crystalline sample. These X-rays are generated by a cathode ray tube, filtered to produce monochromatic radiation, collimated to concentrate, and directed toward the sample. The interaction of the incident rays with the sample produces constructive interference (and a diffracted ray) when conditions satisfy Bragg's Law. This law relates the wavelength of electromagnetic radiation to the diffraction angle and the lattice spacing in a crystalline sample. These diffracted X-rays are then detected, processed and counted. Conversion of the diffraction peaks to d-spacings allows identification of the crystal because each crystal has a set of unique d-spacings. Typically, this is achieved by comparison of d-spacings with standard reference patterns.
X-ray powder diffraction is most widely used for the identification of unknown crystalline materials (e.g. minerals, inorganic compounds). Determination of unknown solids is critical to studies in geology, environmental science, material science, engineering and biology.
Other applications include:
- characterization of crystalline materials
- identification of fine-grained minerals such as clays and mixed
- layer clays that are difficult to determine optically
- determination of unit cell dimensions
- measurement of sample purity
With specialized techniques, XRD can be used to:
- determine crystal structures using Rietveld refinement
- determine of modal amounts of minerals (quantitative analysis)
- characterize thin films samples by: determining lattice mismatch between film and substrate and to inferring stress and strain; determining dislocation density and quality of the film by rocking curve measurements; measuring superlattices in multilayered epitaxial structures; determining the thickness, roughness and density of the film using glancing incidence X-ray reflectivity measurements
- make textural measurements, such as the orientation of grains, in a polycrystalline sample
SEM/EDX

Scanning electron microscopy (SEM) is a method for high-resolution imaging of surfaces. The SEM uses electrons for imaging, much as a light microscope uses visible light. The advantages of SEM over light microscopy include much higher magnification (>200,000X) and greater depth of field up to 100 times that of light microscopy. Qualitative and quantitative chemical analysis information is also obtained using an energy dispersive x-ray spectrometer (EDS) with the SEM. In addition to topographical, morphological and compositional information, a Scanning Electron Microscope can detect and analyze surface fractures, provide information in microstructures, examine surface contaminations, reveal spatial variations in chemical compositions, provide qualitative chemical analyses.
The SEM generates a beam of incident electrons in an electron column above the sample chamber. The electrons are produced by a thermal emission source, such as a heated tungsten filament, or by a field emission cathode (as in our case). The energy of the incident electrons can be as low as 100 eV or as high as 30 keV depending on the evaluation objectives. The electrons are focused into a small beam by a series of electromagnetic lenses in the SEM column. Scanning coils near the end of the column direct and position the focused beam onto the sample surface. The electron beam is scanned in a raster pattern over the surface for imaging. The beam can also be focused at a single point or scanned along a line for x-ray analysis. The incident electrons cause electrons to be emitted from the sample due to elastic and inelastic scattering events within the sample's surface and near-surface material. High-energy electrons that are ejected by an elastic collision of an incident electron, typically with a sample atom's nucleus, are referred to as backscattered electrons. The energy of backscattered electrons will be comparable to that of the incident electrons. Emitted lower-energy electrons resulting from inelastic scattering are called secondary electrons. Secondary electrons can be formed by collisions with the nucleus where substantial energy loss occurs or by the ejection of loosely bound electrons from the sample atoms. The energy of secondary electrons is typically 50 eV or less.
To create an SEM image, the incident electron beam is scanned in a raster pattern across the sample's surface. The emitted electrons are detected for each position in the scanned area by an electron detector. The intensity of the emitted electron signal is displayed as brightness on a display monitor and/or in a digital image file.
SEM/EDX instrument is a powerful and flexible tool for solving a wide range of product and processing problems for a diverse range of metals and materials.
Typical applications include:
- Microscopic feature measurement
- Identification of metals and materials
- Particle contamination identification and elimination
- Classification of materials
- Product and process failure and defect analysis, fracture characterization
- Examination of surface morphology (including stereo imaging) and microstructure
- Analysis and identification of surface and airborne contamination
- Powder morphology, particle size and analysis
- Thin coating evaluations
- Cleaning problems and chemical etching
- Welding and joining technology quality evaluation and failure investigation
- Paint and coating failure and delamination investigation
- Paint, Adhesive, Sealant and Gasket Filler Fingerprinting
- Identification and elimination of corrosion and oxidisation problems
- Contamination or stain investigation
- Structural analysis
- Reverse engineering of products and processes
Range of materials for investigation bt SEM/EDX:
- Metals, Glass and Ceramics
- Semiconductors
- Plastics and polymers
- Powders and Dust
- Composite Materials
- Fibres
XPS

X-ray Photoelectron Spectroscopy (XPS) also known as Electron Spectroscopy for Chemical Analysis (ESCA) is the most widely used surface analysis technique because it can be applied to a broad range of materials and provides valuable quantitative and chemical state information from the surface of the material being studied. The average depth of analysis for an XPS measurement is approximately 5 nm. Although X-Rays can penetrate the sample for several mm, in fact, the measured photoelectron signal originates from a surface depth not more than ~50Å, due to the inelastic scattering of the emitted electrons within the sample. Depth distribution information can be obtained by combining XPS measurements with ion milling (sputtering) to characterize thin film structures. The information XPS provides about surface layers or thin film structures is important for many industrial and research applications where surface or thin film composition plays a critical role in performance including: nanomaterials, photovoltaics, catalysis, corrosion, adhesion, electronic devices and packaging, magnetic media, display technology, surface treatments, and thin film coatings used for numerous applications. The XPS analysis is very important since the characteristics of the surface can heavily differ from the bulk; moreover, surface composition, is deeply affected by the interaction of the materials with environment and can thus be used to investigate catalysis, corrosion, surface oxidation, etc.
X-ray Photoelectron Spectroscopy (XPS), belongs to the wide family of the surface characterization methods based on the detailed energy studies of the electron emitted from a sample surface into ultra-high vacuum environment. XPS is typically accomplished by exciting a samples surface with mono-energetic Al kα or Mg kα x-rays causing photoelectrons to be emitted from the sample surface. An electron energy analyzer is used to measure the energy of the emitted photoelectrons. From the binding energy and intensity of a photoelectron peak, the elemental identity, chemical state, and quantity of a detected element can be determined.
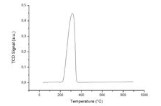
Micromeritics Autochem II 2920

The chemical adsorption isotherm reveals information about the active surface of a material and has been employed for many years as a standard analytical tool for the evaluation of catalysts. In addition, temperature-programmed reaction techniques have emerged as an indispensable companion to chemisorption isotherm analyses in many areas of industry and research. Micromeritics' AutoChem II 2920 Chemisorption Analyzer is a fully automated instrument capable of conducting a comprehensive array of highly precise chemical adsorption and temperature programmed reaction studies. The instrument enables the researcher to obtain valuable information about the physical properties of catalysts, catalyst support, and a variety of other materials. Researchers can investigate active metal surface area, surface acidity, distribution and strength of active sites, BET surface area, and more. The AutoChem II performs pulse chemisorption, temperature-programmed reduction (TPR), desorption (TPD), oxidation (TPO), and reaction analyses. Multiple experiments can be run using the same sample.
GC-MS

Gas Chromatography-Mass Spectrometry (GC-MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances. The instrument is composed by two different blocks: Gas Chromatograph which has the function of separating and analysing compounds, and Mass Spectrometer detector which breaks each molecule into ionized fragments in order to detect that by using their mass/charge ratio. The system could be used in in series; in parallel or stand-alone with the aim of extend the capabilities of measurements. This apparatus can perform accurate determinations of the composition of complex gas mixtures, and is often used for catalytic activity tests. A parallel reactor, which potentially can reach temperatures up to 1000ºC, contains the tested catalyst and controls its temperature while gases are made flown through it. Exhausts are then analyzed by the GC-MS. The instruments available in our laboratory: GC 7890A with thermal conductivity detector (TCD) and MSD 5975C with triple-axis detector, Agilent Technologies.
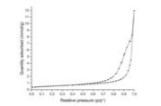
Micromeritics ASAP 2020

The Micromeritics ASAP 2020 Plus is a powerful tool for the measurement of adsorption isotherms, mainly under nitrogen. This technique allows a deep examination of the surface of the materials. Superficial area, porosity, shape of pores, surface energy, are all within the range covered by this instrument. More accurate data can also by obtained by DFT calculations based on isotherms, in order to directly determine the size distribution of the pores. These measurements are particularly useful for porous and high surface materials (>100 m2/g) and gives its best in these cases, but can be useful also for mesoporous and ceramic low area materials. In particular, catalytic activity if often correlated to superficial area, also in high temperature catalysts which tend to have low areas.
Quadrupole Mass Spectrometer
The Quadrupole Mass Spectrometer (QMS) is used in our laboratories to determine the composition of a gas mixture. It is able to separate atomic/molecular species depending on their mass/charge (m/z) ratio, the separation is obtained by imposing an obsillation to the quadrupole which destabilizes the trajectories of all the ions with m/z different from the sampled one. The ions which are not destabiliyzed make a controlled helicoidal trajectory leading to the detector. The high sensitivity ofn this instrument allows to detect gases up to 100ppm of concentration. Being a very compact instrument, can be easily moved and coupled with many of other measurements techniques. At the outlet of a chemisorption analyzer it is employed to verify the chemical species released during desorption anayses. During oxygen semipermeability measurements, the QMS is fundamental for the quantitative determination of the permeated oxygen. It can measure the composition of the exhaust gases from a fuel cell, in order to determine the selectivity of the electrocatalysts towards specific products and to measure the gas consumption efficiency of the device. It can be also used during measurements of catalytic activity, although most of these measurements are carried out with the GC-MS apparatus.
Solid Oxide Cells electrochemical test bench

Tests on Solid Oxide Cells are central in our research. For this purpose, we have a complete bench able to perform most of the common electrochemical measurements typical of this field. We are able to directly determine the temperature at both the electrodes with two dedicated thermocouples, and it is possible also to analyse the composition of the exhaust with the Mass Spectrometer. The composition of the feeding gases is carefully managed with a series of dedicated flowmeters.